How Does Vitamin B12 Work?
Vitamin B12 functions in the body through two bioactive coenzymes:
- Methylcobalamin
Functions in the cell plasma as a coenzyme in the methylation cycle - Adenosylcobalamin
Functions in the mitochondria as a coenzyme in the citric acid cycle
Through these two functional mechanisms, multiple effects take place in diverse biological systems – as explored in our article: Vitamin B12 Benefits
The focus of this article is on methylcobalamin and its working within the methylation cycle.
B12’s vital role in the methylation cycle explains why the vitamin has an effect on areas as diverse as the nervous system, DNA synthesis, detoxification and the synthesis of hormones and neurotransmitters.
This article takes a closer look at the exact biochemical interconnections within the methylation cycle in a way that is easy to read and understand. We believe that knowledge of these mechanisms is key for fully comprehending vitamin B12’s use as a dietary supplement.
Contents:
|
Methylation – An Essential Part of Life
Readers who have already done a little research into vitamin B12 will have probably stumbled across terms such as “methylation”, “methyl group donor” and “methylation cycle”. Yet what exactly do these terms mean? And how do they relate to methylcobalamin?
Methylation reactions are a vital building block of life, crucial for numerous physical processes. In every cell in the body, methylation occurs thousands of times per second; without these methylation reactions, normal cell functions would not be possible.
Vitamin B12 in the form of methylcobalamin plays a central role in methylation, which we will explore in this article. First, however, we need to clarify the basic terms.
What is a Methyl Group?
A methyl group is one of the simplest atomic arrangements in organic chemistry. It consists of only four atoms: a carbon atom with three hydrogen atoms.
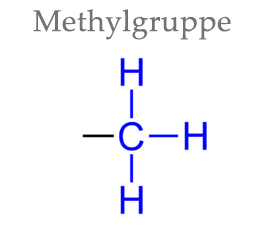
Despite its prevalence, the methyl group is not regarded as an independent chemical substance because it is always present as part of another molecule. Nevertheless, the methyl group is of enormous importance for many bodily processes and plays a central role in many metabolic processes, as we will demonstrate in the following.
What is Methylation?
In very simplified terms, one could say that methylation functions like an on/off switch in many contexts. Both genes and certain molecules can be activated or deactivated by methylation. It is important in many transformation reactions in the body and thus provides a number of important substances.
But what exactly is methylation? Methylation – or more precisely transmethylation – simply means the transfer of methyl groups from one molecule to another. Mediated by certain enzymes, one molecule releases the methyl group and the other absorbs it. This simple reaction often ensures that certain substances are activated and a plethora of metabolic processes can take place.
For methylation to occur, the body depends on a whole range of enzymes that enable the transfer of methyl groups between particular molecules. It is not just one enzyme that facilitates methylation; many different methylation reactions between substances require their own particular enzyme.
What is a Methyl Donor or Methyl Group Donor?
The methyl donor or methyl group donor is the molecule which releases the methyl group during the methylation reaction.
Methyl donors are essential because methyl groups are constantly needed throughout the body for a wide variety of processes. Certain substances act as universal methyl donors in the body and thereby play a central role in countless biological functions; they ensure that all physical processes are constantly supplied with sufficient methyl groups. In principle, these substances can be closely compared to adenosine triphosphate (ATP): the universal energy source of the body.
The most important methyl donor in humans is S-adenosylmethionine (SAM), which we will take a closer look at below (1).
SAM is produced within the body and obtains its required methyl group from methylcobalamin – underlining the importance of methylcobalamin as a central supplier of methyl groups.
In the case of vitamin B12 deficiency, SAM cannot be formed. Vitamin B12 is therefore of paramount importance to the vital methylation process.
What are the Positive Effects of Methylation?
While methylation plays a role in many central processes, its most important functions are:
Controlling DNA/epigenetics
Methylation is utilised in the body to switch genes off and on. This does not change the information of the genetic material itself, but only whether certain genes are read and how. About 60% of human DNA is methylated and thus epigenetics has a profound effect on health (2-4).Regeneration of DNA and RNA
Methylation functions are also necessary for the repair and regeneration of DNA and RNA, as the required purines and pyrimidines are formed by the methylation cycle. Since millions of cells have to be replaced every minute, this is of paramount importance. Mistakes in the formation of DNA and RNA can lead to severe diseases (5-7).Immune function
Monocytes and lymphocytes, two types of immune cells, must be methylated in order to become active. This demonstrates how parts of the adaptive immune system depend on epigenetic mechanisms (8-10).Synthesis and regulation of neurotransmitters and hormones
The synthesis of diverse hormones and neurotransmitters, including all monoamine neurotransmitters, are reliant on the methylation cycle. Interrupted methylation is today seen as a main factor in many psychological illnesses (11 – 13).Detoxification
Through methylation, heavy metals become water-soluble and can be excreted from the body. At the same time, the methylation cycle is involved in the formation of glutathione, one of the body’s most important detoxifiers and radical scavengers (14 – 17).Energy
The production of the coenzyme Q10 and carnitine depends on methylation (19, 20).Protein methylation
Various central proteins depend on methylation: to be produced in the first place and/or to carry out certain functions. This has an effect on various signalling pathways in cells, DNA repair, the formation of the myelin nerve fibre layer and numerous other areas. It thus affects almost all aspects of health (21).Phospholipid methylation
The phospholipids play a role in the body that is almost as important as proteins. They are the main component of the cell membrane and hence determine the function of each individual cell as well as the communication between cells. The phospholipids are also dependent on methylation (22 – 24).
Vitamin B12 and the Methylation Cycle
The majority of methylation processes in the body depend on S-adenosylmethionine (SAM) as the methyl donor. SAM is regenerated again and again in a permanent cycle: it releases its methyl group in various reactions, and then goes through a series of reaction steps, through which it receives a new methyl group – and is thus available again as a methyl donor. This continuous cycle of SAM regeneration is known as the methionine cycle.
The methionine cycle is often referred to as the methylation cycle, but this is not entirely correct. Depending on where one draws the line, a total of two to four different cycles are involved in the methylation process, all of which are closely interlinked. Only when all of the interactions between these cycles are taken into account, can a clear picture of the significance of these metabolic processes be gained.
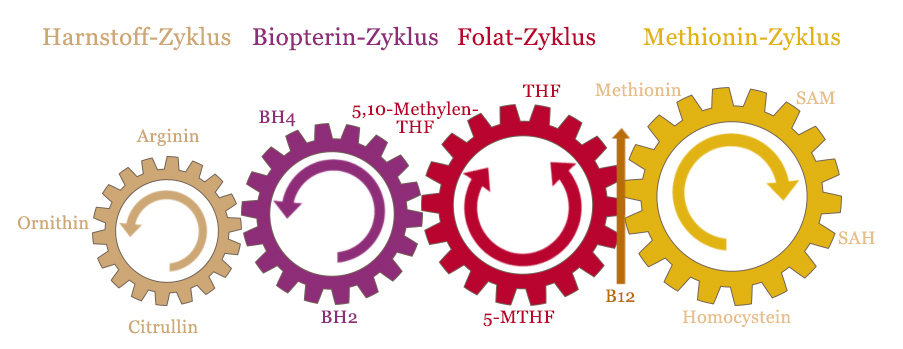
This graphic shows the four interlocking cycles in the methylation cycle.
As you can see, vitamin B12 is the junction between the methionine cycle and the folate cycle. This is the central intersection in the regeneration of SAM by homocysteine.
Vitamin B12 is a critical factor in the methylation cycle; without it, the entire system comes to a standstill. A sufficient supply of B12 is therefore the first step in optimising the methylation processes.
Methylcobalamin for Methylation
The active form of vitamin B12 in the methylation cycle is called methylcobalamin. Due to the importance of the methylation cycle for health, methylcobalamin is the most popular vitamin B12 form used in supplements today.
Vitamin B12 supplements should contain methylcobalamin to optimise methylation. However, it is best when mixed with the coenzyme adenosylcobalamin (which we discuss in our article: Vitamin B12 Supplements).
Key Points:
|
Suitable dosage of B12 supplements for methylation
| Active Ingredient | Dose | Online Search |
| Methylcobalamin | 1000 µg | Methylcobalamin + B12 + bioactive + 1000 µg |
| Methylcobalamin + Asdenosylcobalamin | 1000 µg | Methylcobalamin + adenosylcobalamin + bioactive + 1000 µg |
For further, general information on vitamin B12 supplement dosages, click here.
Vitamin B12 and Methylfolate (5-MTHF)
Vitamin B12 works in the methylation cycle together with methylfolate (5-MTHF). In cases of folate deficiency, a combination of these two vitamins can bring remarkable improvement to many negative health symptoms.
Folate should not be confused with folic acid. While all naturally occurring folates have an effect in the body, synthetic folic acid (chemically: pteroylmonoglutamic acid) has no vitamin function of its own. It must first be transformed in the body to become effective.
The effective form of folate is called methylfolate (5-MTHF). As an isolated active ingredient in supplements it is quite unstable and must therefore be bound to natural stabilisers such as calcium or glucosamine in order to show sufficient bioavailability. Currently, the active ingredient with the highest bioavailability is Quatrefolic.
Key Points:
|
Common dosage for general supplementation (for simultaneous B12 and folic acid deficiency)
| Nutrients | Dose | Online Search |
| Vitamin B12 + methylfolate (L-5-MTHF, Quatrefolic) | 1000 µg + 400 µg | Methylcobalamin + adenosylcobalamin + 1000µg + Quatrefolic folate 400 µg |
In the following, various parts of the methylation cycle will be examined in detail before we build an overall picture.
The Methionine Cycle
As indicated above, the methionine cycle is the central aspect of the methylation cycle and is therefore sometimes referred to as the methylation cycle alone.
The methionine cycle is responsible for the synthesis of SAM and thus for the majority of all methylation reactions in the body. It influences several vital biological areas, such as the health of the nerves, the methylation of proteins, the structure of cell membranes and the activation of certain neurotransmitters.
The sign of methionine cycle malfunction is an increased level of homocysteine, which many doctors today measure as an important clinical parameter in blood tests.
The methionine cycle consists of 4 substances:
- Methionine
- S-adenosylmethionine (SAM)
- S-adenosyl homocysteine (SAH)
- Homocysteine
The essential task of this cycle is to break down harmful homocysteine and to regenerate it again into the methyl donor S-adenosylmethionine (SAM). As described above, SAM is the most important universal methyl group donor in the human body.
The limiting step of this cycle is the conversion of homocysteine to methionine by the enzyme methionine synthetase (MS or MTR). The necessary reaction requires vitamin B12 in the form of methylcobalamin as a cofactor. Methylcobalamin, however, only occurs as a volatile intermediate product: reduced cobalamin (without a side group) accepts the methyl group of methylfolate (5-MTHF) and passes it on to homocysteine, producing methionine.
Ultimately, vitamin B12 leaves this reaction unchanged. Over time, however, there is always a certain amount of inactive cob(II)-cobalamin produced, which must be reactivated through methylation into methylcobalamin with the help of a special enzyme. Here SAM acts as a methyl donor – which is actually formed with the help of vitamin B12. In order to minimise the amount of SAM that is lost in this process, methylcobalamin is today obtained directly in supplements. As a result, plenty of methyl groups are available and cobalamin recycling via SAM is superfluous.
For more details on homocysteine, see the article: Homocysteine and Vitamin B12
The Steps of the Methionine Cycle
Step 1: methionine is converted into SAM by the enzyme methioninadenosyltransferase (MAT) in the presence of magnesium (Mg) and ATP (universal energy donor).
Step 2: SAM releases its methyl group in various reactions as a methyl donor and is thus converted into SAH.
Step 3: SAH in turn is converted to homocysteine by the enzyme S-adenosylhomocysteine hydrogenase (SAHH), which also produces adenosine. Homocysteine is a neurotoxic and vascular-damaging amino acid, which today is regarded by many as a cytotoxin. On one hand, adenosine is part of RNA; but on the other, it causes drowsiness. Caffeine, for example, produces its stimulating effect by inhibiting the adenosine receptors. If the cycle is stuck at this point, this leads to a lack of SAM, as well as to many other health problems due to the accumulation of homocysteine.
Step 4: Here there are three possible ways through which homocysteine can be degraded or reactivated into methionine.
a) Methionine synthase: we have already briefly discussed this possibility above, where homocysteine is converted back into methionine, which is dependent on vitamin B12 and the folate cycle. This is the point at which the methionine and folate cycles meet, making it clear that they cannot really function without each other.
b) Transsulfuration: homocysteine is converted into cystathion by the enzyme cystathion B synthase (CBS) and the presence of cofactors vitamin B6 and haem. Then, cystathion in turn is converted into cysteine and alphaketoglutarate. This is the starting material for important substances such as taurine and the central detoxifier, glutathione.
c) BHMT: The third possibility for this step takes place almost exclusively in the liver. Here, homocysteine is converted back into methionine independently of B12 and the folate cycle, with trimethylglycine (TMG, also called betaine = methylated choline) functioning as a cofactor of the enzyme betaine homocysteine methyltransferase (BHMT). In this case, the methyl group does not come from methylcobalamin, but from TMG, which turns it into dimethylglycine (DMG).
All of these steps have an important significance, as indicated above, but the key aspect is perhaps the step via vitamin B12-dependent methionine synthase – which links the entire methionine cycle directly to the folate cycle.
The Folate Cycle
The folate cycle actually consists of two different cycles: one is a large cycle involved in methylation, and the other is a small cycle involved in the formation of various substances that play a role in the synthesis of DNA.
The Large Folate Cycle
The large folate cycle consists of three substances:
- Tetrahydrofolate (THF)
- 5, 10-Methylenetetrahydrafolate
- Methylfolate (5-MTHF)
The folate cycle is responsible for converting tetrahydrofolate (THF) into initially 5, 10-methylenetetrahydrafolate and finally methylfolate (5-MTHF). 5-MTHF gives off its methyl group to the methionine cycle, which produces THF again.
The Small Folate Cycle
An important alternative cycle exists: from 5, 10-methylenetetrahydrofolate via dihydrofolate (DHF) to THF. Here, the important building blocks for DNA synthesis – purines and pyrimidines – are produced. For the sake of simplicity, we will not go into further details here about this cycle.
The Steps of the Large Folate Cycle
We will now take a quick look at the large folate cycle starting from THF. Note that this cycle in the graph is anticlockwise.
Step 1: THF is converted into 5, 10-methylenetetrahydrofolate with the help of vitamin B6 and serine. THF receives a “methylene group” (not a methyl group) of serine. As an alternative to THF, folinic acid (5-formyltetrahydrofolate) can also be converted into 5, 10-methylenetetrahydrofolate. Artificial folic acid, which is contained in many dietary supplements, cannot be used directly. It must first be converted into DHF with the aid of vitamin B3 and then into THF – also with the aid of vitamin B3.
Step 2: 5, 10-methylenetetrahydrofolate is converted into methylfolate (5-MTHF) by the enzyme methylenetetrahydrafolate reductase (MTHFR) with the aid of NADH, B2 and ATP.
Step 3: MTHF releases its methyl group via vitamin B12 to the methionine cycle, which produces THF again and restarts the cycle.
Further reading: Vitamin B12 and Folic Acid
The MTHFR Gene
The critical stage in the folate cycle is the conversion of 5, 10-methylenetetrahydrofolate to 5-MTHF, which is then used in the methionine cycle. This conversion is dependent on the enzyme MTHFR. Many people have a mutation in the gene responsible for this. Depending on the type of mutation, the efficacy of the enzyme can be reduced by up to 70%.
This results in a deficiency of MTHF, which in turn leads to a deficiency of SAM; affecting almost all methylation reactions in the body.
Between 10 – 30% of the population have a mutation in the MTHFR gene and it is becoming increasingly clear that this could have pronounced medical implications. Especially since MTHFR has an important dual function, as we will explore below.
The Biopterin (BH4) Cycle
The biopterin cycle consists of only two substances:
- Tetrahydrobiopterin (BH4)
- Dihydrobiopterin (BH2)
BH4 also acts as a cofactor in various chemical reactions, where it becomes BH2. This is then regenerated into BH4 with the help of 5-MTHF from the folate cycle. This demonstrates the dual function of 5-MTHF and its enzyme, MTHFR. MTHFR is not only important for the formation of 5-MTHF (and thus the methionine cycle) but also for the regeneration of BH4.
Tetrahydrobiopterin (BH4) is of central importance for the normal functioning of the central nervous system. It is an essential cofactor for the enzymes involved in the synthesis of vital hormones and monoamine neurotransmitters, in particular catecholamines (dopamine, adrenaline, noradrenaline) and indolamines (serotonin and melatonin). The BH4 level determines the production rate of these important hormones and neurotransmitters and thus the body’s ability to synthesise neurotransmitters such as dopamine, adrenaline and serotonin.
Moreover, BH4 is important for all three forms of nitric oxide synthase (NOS) and thus influences the adjacent urea cycle, which we will not discuss here.
The biopterin cycle is responsible for the synthesis of two essential starting substances for hormones and neurotransmitters:
- 5-HTP (serotonin, melatonin)
- L-dopa (dopamine, adrenaline, noradrenaline)
We will now take a closer look at these two substances.
Serotonin Synthesis
BH4 is a cofactor in the conversion of tryptophan into 5-hydroxytryptophan (5-HTP). 5-HTP is converted into serotonin by the enzyme hydroxytryptophan decarboxylase and with the help of vitamin B6.
Serotonin can then be converted into melatonin with the help of a SAM-dependent methylation reaction – this again shows an interconnection to the methionine cycle. This compound may also explain why vitamin B12 has been shown to be extremely helpful for sleep disorders, as melatonin regulates the sleep-wake cycle.
Dopamine Synthesis
A second reaction in which BH4 acts as a cofactor is the conversion of tyrosine to L-dopa, which is further metabolised to dopamine.
Dopamine can be further converted into noradrenaline with the help of vitamin C, and this in turn can be metabolised to adrenaline by a methylation reaction (again dependent on SAM).
Degradation of Neurotransmitters by MAO and COMT
SAM is also used to degrade neurotransmitters via monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT); a process that is as important for signal transmission as synthesis. An excess of neurotransmitters can be just as undesirable as a deficiency.
Inhibitors of these enzymes are administered to treat various mental illnesses, whereas deficiency is associated with different clinical pictures.
A Web of Interconnections
The above diagram is still incredibly simplified and omits many further interconnections; hopefully however it shows how closely linked the different cycles are and what a broad significance they have altogether.
It also shows how interruptions at individual sites – such as vitamin B12 deficiency or MTHFR mutations – can block the entire system, resulting in negative health symptoms in a wide range of areas.
Only an understanding of the methylation cycle allows for a deeper comprehension of the many effects of vitamin B12 on areas as diverse as nerves, DNA synthesis and neurotransmitters.
Under- and Over-Methylation
The SAM level resulting from the methylation cycle may be the cause of both under- and over-methylation.
Under-methylation
Under-methylation occurs when the synthesis of SAM in the methionine cycle is disturbed. Resultantly, too little SAM is formed and the body lacks methyl groups for various reactions.
Over-methylation
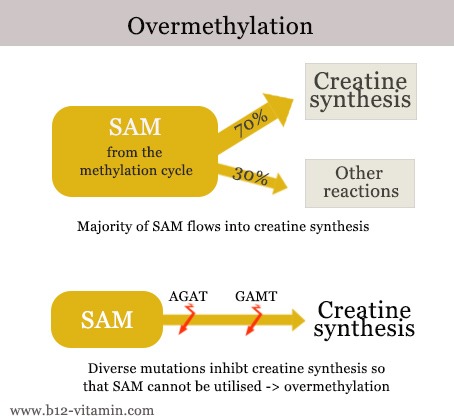
Over-methylation occurs when there is too much SAM in circulation. This can happen due to taking the wrong dosage of dietary supplements, but also because SAM cannot be utilised – due to genetic mutations, for instance. About 70% of SAM normally flows into creatine synthesis. Creatine is an important substance for the building and functioning of muscles. If creatine synthesis is disturbed – e.g. by mutations in the AGAT or GAMT genes – SAM cannot be utilised and circulates at elevated levels.
How can the Methylation Status be Measured?
There is currently no guaranteed method for measuring the status of methylation, but there are some good markers.
Histamine (in blood)
The methyl donor SAM is necessary for the degradation of histamine by the enzyme HMNT. If methylation is defective, the histamine blood level rises. However, this marker is not completely clear because HMNT is only responsible for about 70% of histamine degradation – the rest is done by the enzyme DAO. In addition, gene mutations in these degradation enzymes can falsify the result. If the histamine is extremely high, there is still a strong likelihood of under-methylation; if it is extremely low, good methylation can be assumed.
Ratio of SAM to SAH
Tests on the ratio of S-adenosylmethionine (SAM) to its degradation product S-adenosylhomocysteine (SAH) are only available in a few laboratories and from doctors. If the ratio is strongly shifted towards SAH, there is under-methylation.
Homocysteine
Homocysteine increases with malfunctional methylation. The cause is either a lack of vitamin B12 or folic acid.
Vitamin B12 and folic acid
Both nutrients have a major influence on the methylation cycle and should be measured if a methylation disorder is suspected. (See here: Vitamin B12 Deficiency Test)
Interruptions in the Methylation Cycle
The methylation cycle can be interrupted at various points, either due to nutrient deficiencies or genetic mutations which affect the required enzymes.
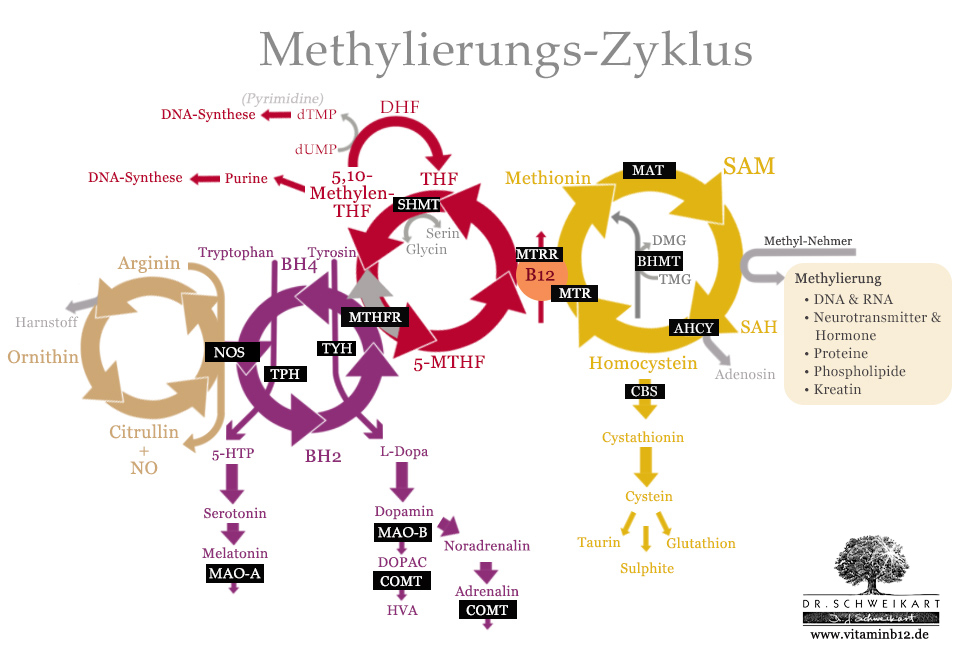
The most important genes and enzymes in the methylation cycle (highlighted in black).
How Useful are Gene Tests?
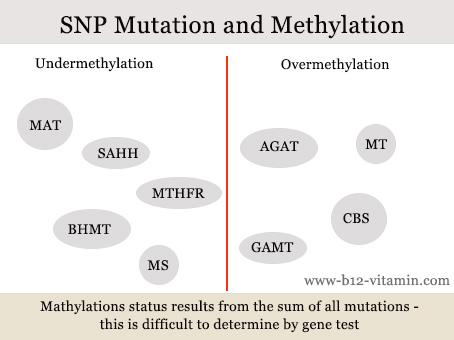
Gene mutations – known as single nucleotide polymorphisms (SNPs) – play a major role in the methylation cycle. Plus, many people have several such mutations at the same time. In this case, it is difficult to predict the overall effect, even with a gene test.
A number of mutations promote under-methylation, another group over-methylation.
In our opinion, it therefore makes more sense to measure the levels of the nutrients and metabolites involved than to undertake a gene test.
The Key Factors in the Methylation Cycle
We will now consider three important causes of interruption to the methylation cycle:
- Vitamin B12 deficiency
- Folate deficiency
- MTHFR mutation
Vitamin B12 deficiency
If vitamin B12 is deficient, the methyl group cannot be transferred from methylfolate to the methionine cycle.
As a result, the folate cycle is at the 5-MTHF stage. 5-MTHF accumulates in what is known as the “folate trap”. Even the small folate cycle can only proceed to a limited extent because the supply of THF from the large folate cycle is no longer given. Plus any folate that is ingested in the diet is sooner or later also stuck in the “folate trap”. This leads to anaemia and cell damage.
The methionine cycle also comes to a standstill, the level of SAM decreases drastically and the harmful homocysteine increases. This affects all SAM-dependent reactions, for example the nerves – through reduced production of myelin and acetylcholine – but also neurotransmitter synthesis and degradation in the biopterin cycle, as we discussed above.
Folate deficiency
In the case of a folate deficiency, the entire folate cycle comes to a standstill – with corresponding consequences on the methionine cycle and the biopterin cycle. Due to the mediating position of the folate cycle between the two adjacent cycles, a folic acid deficiency has widespread consequences: the production of SAM falls, as does that of BH4 and the associated neurotransmitters.
At the same time, the small folate cycle is also affected, which has strong effects on cell division and the formation and repair of DNA. This leads to cell damage and anaemia.
MTHFR mutation
We have already touched on MTHFR mutation. Here the folate cycle of 5, 10-methylenetetrahydrafolate is stuck and as a result can produce little to no 5-MTHF. This in turn halts both the methionine cycle and the biopterin cycle. In contrast to folate deficiency, the small folate cycle can run almost as normal.
What is mainly affected here is the synthesis of neurotransmitters and also various methylation reactions, due to a SAM deficiency.
Methylation and Epigenetics
Methylation has a major influence of epigenetics, an important regulatory mechanism of our genetic material. Every cell in the body contains identical genetic material. About 20 000 genes are known to encode the information for specific proteins. Various organs and tissues require many different combinations of proteins to both form and function.
To this end, different tissues and organs have what are known as epigenetic markers within the genome: they work just like bookmarks indicating which genes are to be read and how. What is more, these markers can change throughout life, which can have both positive and negative effects. Today it is assumed that numerous diseases could be caused by epigenetic errors.
There are two important epigenetic mechanisms – methylation plays a central role in both:
- DNA methylation
The switching on and off of particular genes through methylation. - Histone modification
The opening and closing of specific areas of the DNA through methylation or acetylation.
Methylation and diverse nutrients have a significant impact on histone modification. DNA is located in the cell nucleus, in a densely packed, rolled up state. Histones are basic proteins that can be imagined as coils around which DNA is wound.
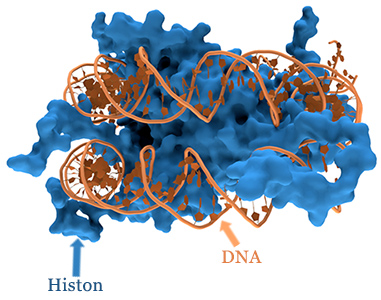
In order for a gene to be read, the DNA must first be unrolled from this histone coil. Methyl- and acetyl-groups determine how successful this is: methyl-groups close the DNA, whilst acetyl-groups open it. The relationship between methyl- and acetyl-groups is changed by a disruption to methylation, which can have a direct effect on particular areas of the genetic material.
Epigenetics and Methylation: Effects on the Psyche
One area that is particularly effected are the transmembrane proteins of the neurotransmitters. They are responsible for the reabsorption (deactivation) of the neurotransmitters; they act like sponges, absorbing and removing the neurotransmitters from the synapses.
The activity of these reabsorption proteins depends essentially on the gene expression of certain regions of the DNA. Whether these can be read depends on the concentration of methyl and acetyl groups. This in turn is reliant on certain enzymes (acetlyases and methylases), which are directly controlled by various nutrients, in particular the B vitamins.
Vitamin | Effect | Use |
Niacin (vitamin B3) | Reduces dopamine activity | Anxiety disorders, bipolar disorders, schizophrenia |
SAM | Encourages serotonin activity (= serotonin reabsorption inhibitor) | Depression |
Folate, Folic acid | Encourages the production of serotonin, dopamine and adrenalin in the methylation cycle, at the same time inhibits their receptor activity through epigenetic mechanisms | Helpful for some forms of depression, negative for others |
For more information on how vitamin B12 can effect the brain and psyche, click here.
The Methylation Cycle in Nutrient Therapy
The decoding of the methylation cycle and the understanding of the above-mentioned blockages has led to numerous therapeutic approaches aimed at optimising methylation and restoring the interaction of the four cycles.
Among other things, these approaches have given new impulses for nutrient medicine and enabled targeted therapeutic supplementation through a deeper understanding of the interrelationships.
More recently, various mutations have been discovered that influence the central enzymes of the methylation cycle – the MTHFR mutation has already been discussed in this context. Today, targeted gene tests make it possible to diagnose exactly where the methylation cycle is interrupted and thus enable specific therapy with the corresponding nutrients.
Folic Acid: A Double-Edged Sword for Depression?
Methylfolate is recommended for various uses. Folate is indeed one of the central nutrients in the methylation cycle and a deficiency can have serious effects – especially for the synthesis of important hormones and neurotransmitters such as serotonin
Treatment with vitamin B12 and methylfolate can produce extremely good results for people who do not respond to regular antidepressants. The reason is that these antidepressants act as serotonin reabsorption inhibitors – preventing serotonin from being rendered ineffective by the body. This is not successful in the case of folate deficiency because the body can hardly produce serotonin. Methylfolate produces rapid improvement here (26 – 28).
This is not the case for a certain percentage of patients because folate acts in two different ways:
- As part of the methylation cycle
- As an epigenetic factor
We have already explored the role of the methylation cycle.
In epigenetics, folate has a demethylating effect (25). By means of the enzyme lysine-specific demethylase (LSD-1), it ensures the demethylation of histones, whereby some genes are increasingly expressed. This probably also includes the serotonin transporter SERT, which inhibits the effect of serotonin.
In the case of under-methylation, this could lead to paradoxical results. Although methylfolate increases the formation of serotonin, it also promotes the expression of SERT through the demethylation of histones that are already methylated, thereby inhibiting the function of serotonin.
In case of poor results with methylfolate, either mega doses of methlfolate should be given (up to 15 mg were used in studies) to provide sufficient methyl groups – or SAM can be given directly.
Depression is a symptom with many causes. Depending on the type of depression, methylfolate can either bring dramatic improvements or cause problems.
Dietary Supplements for Optimising the Methylation Cycle
Nutritional supplements can be administered in two ways:
To optimise the methylation cycle
Here nutrient deficiencies are rebalanced to allow the cycle to function smoothlyTo avoid interruptions
Genetic mutations, which have a negative effect on/interrupt the methylation cycle, can also be circumvented with the help of nutrients
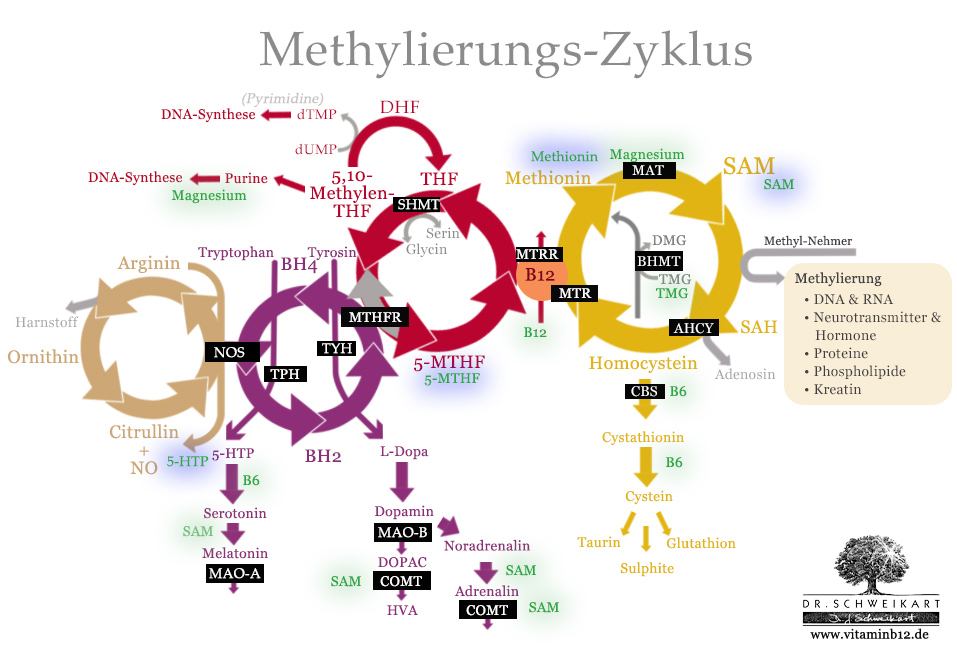
Important nutritional supplements for the optimisation of the methylation cycle (green) and in the case of genetic mutation (blue).
Nutrients for Optimisation
The most important nutrients for the optimisation of the methylation cycle are:
- Vitamin B12 (as hydroxo- or methylcobalamin)
- Methylfolate (as 5-MTHF, Quatrefolic)
- Vitamin B6 (as pyridoxal-5-phosphate)
- Magnesium
Nutrients for Therapy
The following substances are administered for genetic mutations:
- Methylfolate (as 5-MTHF, Quatrefolic)
Bypasses the MTHFR mutations - Methionine
Bypasses problems with MTHFR - SAM
Bypasses problems with MTHFR and MAT - 5-HTP
Bypasses BH4 deficiency or TPH mutations
Nutrient Combinations for Certain Symptoms
The following nutrient combinations are possible for certain symptoms:
| Symptom/Purpose | Nutrient |
| General supplementation | Vitamin B12 (as methylcobalamin or a mix) + folate (Quatrefolic) |
| Depression | Vitamin B12 (methylcobalamin or a mix) + folate (Quatrefolic) + Vitamin B6 (as pyridoxal-5-phosphate) or 5-HTP + SAM + vitamin B6 + TMG (betaine) |
| Sleep disorders | Vitamin B12 (methylcobalamin or a mix) + Quatrefolic + vitamin B6 (as pyridoxal-5-phosphate) |
| Increased homocysteine | Vitamin B12 (as methylcobalamin or a mix) + folate (as Quatrefolic) + vitamin B6 (as pyridoxal-5-phosphate) + TMG (betaine) |
| MTHFR mutation | Vitamin B12 (as methycobalamin or a mix) + folate (as Quatrefolic) |
Nutrients for MTHFR Mutation
MTHFR mutation is by far the most common genetic mutation. If present, the conversion to 5-methyltetrahydrofolate is disturbed. Direct supplementation with 800 µg 5-MTHF and 1000 µg vitamin B12 usually produces good results.
A supplementation with methylfolate alone is not always effective, as it has to interact closely with vitamin B12. A combination of both vitamins therefore produces the best outcomes.
5-MTHF is only partially bioavailable because it is partly destroyed in the stomach. However, there are two patented active ingredients that stabilise the methylfolate and thus drastically increase bioavailability.
Currently, the best active ingredient is Quatrefolic.
Stabiliser | Availability | |
Metafoline | Calcium | Good |
Quatrefolic | Glucosamine | Very good, 20% better availability than Metafoline |
Conclusion
In recent years, the targeted supplementation of nutrients to optimise methylation processes has proven to be an exciting therapeutic option. It has produced successful results for a wide range of conditions: from psychological to neurological to cardiovascular disorders.
Only an intricate understanding of the complex interconnections of different biological processes enables a targeted approach, leading to correspondingly better outcomes.
In this article, we hope to have provided an accessible insight into the exciting world of nutrient medicine and to have enabled a better understanding of the effects of vitamin B12.
Sources
- Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP. S-Adenosylmethionine and methylation. FASEB J. 1996 Mar;10(4):471-80. Review.
- Holliday, R. (2006). Epigenetics: a historical overview. Epigenetics, 1(2), 76-80.
- Bird, A. (1992). The essentials of DNA methylation. Cell, 70(1), 5-8.
- Jones, P. A., & Takai, D. (2001). The role of DNA methylation in mammalian epigenetics. Science, 293(5532), 1068-1070.
- Van Der Weyden, M. B., Cooper, M., & Firkin, B. G. (1973). Defective DNA Synthesis in Human Megaloblastic Bone Marrow: Effects of Hydroxy-B12 5′-Deoxyadenosyl-B12 and Methyl-B12. Blood, 41(2), 299-308.
- Metz, J., Kelly, A., Swett, V. C., Waxman, S., & Herbert, V. (1968). Deranged DNA Synthesis by Bone Marrow from Vitamin B12-Deficient Humans. British journal of haematology, 14(6), 575-592.
- Hoffbrand, A. V., & Jackson, B. F. A. (1993). Correction of the DNA synthesis defect in vitamin B12 deficiency by tetrahydrofolate: evidence in favour of the methyl-folate trap hypothesis as the cause of megaloblastic anaemia in vitamin B12 deficiency. British journal of haematology, 83(4), 643-647.
- Scharer CD, Barwick BG, Youngblood BA, Ahmed R, Boss JM. Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol. 2013 Sep 15;191(6):3419-29.
- Yi, P., Melnyk, S., Pogribna, M., Pogribny, I. P., Hine, R. J., & James, S. J. (2000). Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. Journal of Biological Chemistry, 275(38), 29318-29323.
- Jacob, R. A., Gretz, D. M., Taylor, P. C., James, S. J., Pogribny, I. P., Miller, B. J., … & Swendseid, M. E. (1998). Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. The Journal of nutrition, 128(7), 1204-1212.
- Bottiglieri, T., Laundy, M., Crellin, R., Toone, B. K., Carney, M. W., & Reynolds, E. H. (2000). Homocysteine, folate, methylation, and monoamine metabolism in depression. Journal of Neurology, Neurosurgery & Psychiatry, 69(2), 228-232.
- Miller, A. L. (2008). The methylation, neurotransmitter, and antioxidant connections between folate and depression. Alternative Medicine Review, 13(3), 216-227.
- Regland, B., Gottfries, C. G., & Oreland, L. (1991). Vitamin B12-induced reduction of platelet monoamine oxidase activity in patients with dementia and pernicious anaemia. European archives of psychiatry and clinical neuroscience, 240(4-5), 288-291.
- Ling, C. T., & Chow, B. F. (1953). The effect of vitamin B12 on the levels of soluble sulfhydryl compounds in blood. Journal of Biological Chemistry, 202, 445-456.
- Mosharov, E., Cranford, M. R., & Banerjee, R. (2000). The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry, 39(42), 13005-13011.
- Kikuchi, M., Kashii, S., Honda, Y., Tamura, Y., Kaneda, K., & Akaike, A. (1997). Protective effects of methylcobalamin, a vitamin B12 analog, against glutamate-induced neurotoxicity in retinal cell culture. Investigative ophthalmology & visual science, 38(5), 848-854.
- Robert A. Zakharyan, H.Vasken Aposhian, Arsenite Methylation by Methylvitamin B12and Glutathione Does Not Require an Enzyme, Toxicology and Applied Pharmacology, Volume 154, Issue 3, 1999, Pages 287-291, ISSN 0041-008X
- Ridley, W. P., Dizikes, L. J., & Wood, J. M. (1977). Biomethylation of toxic elements in the environment. Science, 197(4301), 329-332.
- Brass, E. P., & Stabler, S. P. (1988). Carnitine metabolism in the vitamin B-12-deficient rat. Biochemical journal, 255(1), 153-159.
- Quinzii, C. M., DiMauro, S., & Hirano, M. (2007). Human coenzyme Q10 deficiency. Neurochemical research, 32(4-5), 723-727.
- Kim, S., Lim, I. K., Park, G. H., & Paik, W. K. (1997). Biological methylation of myelin basic protein: enzymology and biological significance. The international journal of biochemistry & cell biology, 29(5), 743-751.
- Hirata, F., & Axelrod, J. (1980). Phospholipid methylation and biological signal transmission. Science, 209(4461), 1082-1090.
- Hirata, F. (2013). Regulation of membrane fluidity by phospholipid methylation. Membrane Fluidity in Biology: Cellular Aspects, 247.
- Vance, D. E. (2014). Phospholipid methylation in mammals: from biochemistry to physiological function. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1838(6), 1477-1487.
- Luka Z, Pakhomova S, Loukachevitch LV, Calcutt MW, Newcomer ME, Wagner C. Crystal structure of the histone lysine specific demethylase LSD1 complexed with tetrahydrofolate. Protein Sci. 2014 Jul;23(7):993-8.
- Godfrey, P. S. A., Toone, B. K., Bottiglien, T., Laundy, M., Reynolds, E. H., Carney, M. W. P., … & Chanarin, I. (1990). Enhancement of recovery from psychiatric illness by methylfolate. The Lancet, 336(8712), 392-395.
- Papakostas, G. I., Shelton, R. C., Zajecka, J. M., Etemad, B., Rickels, K., Clain, A., … & Pencina, M. (2012). L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. American Journal of Psychiatry, 169(12), 1267-1274.
- Stahl, S. M. (2007). Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS spectrums, 12(10), 739-744.