The Various Forms of Vitamin B12
Vitamin B12 is the most chemically complex of all the vitamins. Its chemical name is “cobalamin”, derived from its central cobalt atom. The impressive formula – C63H88N14O14PCo – gives an idea of the size of the molecule that constitutes vitamin B12. Nonetheless, cobalamin almost never occurs in its chemically pure form, but is usually bound to other molecules. These different binding partners determine the names of the resultant vitamin B12 forms.
Vitamin B12 Forms in Food
In foods the most common vitamin B12 forms are:
Adenosylcobalamin and hydroxocobalamin are the most frequently occurring forms in meat, whilst methylcobalamin is especially found in dairy. Other forms of vitamin B12 are very rarely found in food products – and even then only in minor traces. Moreover, it is very rare that any forms of vitamin B12 are found in plant-based foods, making a dietary supply of B12 difficult for vegans to obtain (see here: Vitamin B12 for Vegetarians and Vegans).
Vitamin B12 Forms in the Body
In the body, absorbed B12 works as a coenzyme (further info: Vitamin B12 Benefits), which supports the functions of a multitude of important enzymes. Only two forms of B12 are active as a coenzyme in the body:
Methylcobalamin and adenosylcobalamin are the active coenzyme forms of B12. Methylcobalamin works in the cell plasma, whilst adenosylcobalamin is only active in the mitochondria.
Hydroxocobalamin (a.k.a. hydroxycobalamin) is not itself a coenzyme form of B12, yet can easily be converted by the body into both methyl- and adenosylcobalamin, and is a common transitional form in the B12 metabolism. Furthermore, it binds particularly well to the body’s transport molecules and thus circulates for a long time in the blood – this makes it the best storage form of the vitamin.
- In all tissues (muscles, organs – especially the liver) mainly adenosylcobalamin is found
- In the blood and spinal cord methylcobalamin and hydroxocobalamin are found in equal measure (10)
- In the cells both adenosylcobalamin and methylcobalamin are required, which can easily be transformed into each other
Vitamin B12 Active Ingredients in Supplements
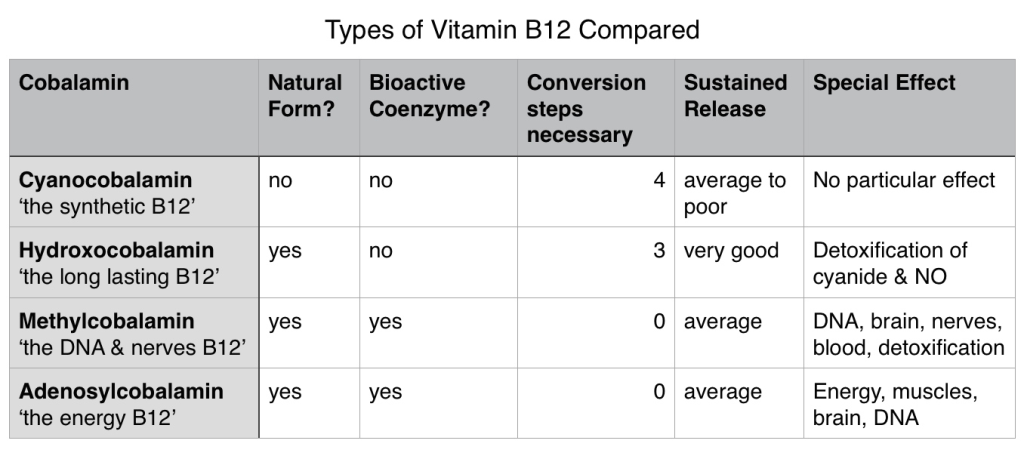
Previously, in vitamin B12 supplements synthetic cyanocobalamin and hydroxocobalamin were mainly used in B12 injections. Since the benefits of hydroxocobalamin over cyanocobalamin have become more apparent, the former has become the main substance used in B12 injections in Europe. Some researchers even think that cyanocobalamin should be completely withdrawn from the market (1).
Likewise in oral supplements such as tablets and capsules, cyanocobalamin is still the most used active ingredient. Although methylcobalamin and adenosylcobalamin are the bioactive forms of B12, they are unfortunately very chemically unstable outside of the body – mainly due to their photosensitivity – and therefore more difficult to produce.
Recently, however, methylcobalamin and adenosylcobalamin have become increasingly used in supplements because of their clear therapeutic advantages (see below).
The Best Vitamin B12 Supplement is a Mixture
When it comes to supplements, the ideal content is a mixture of all the natural B12 forms, as the body urgently needs each one for different tasks. The two active forms are produced on separate metabolic pathways and perform completely different functions. Whilst for a long time an intake of methylcobalamin alone was considered sufficient, today this has been increasingly drawn into question (11).
Instead of taking a single active ingredient, a combination of all of the natural B12 forms is the optimal solution – as it mirrors the B12 content in foods.
The ideal B12 supplement contains a mix of methylcobalamin, hydroxocobalamin and adenosylcobalamin (11).
For details on how much of a supplement to take, see the article: Vitamin B12 Dosages. Plus, to learn more about how to check your vitamin B12 status, see: Vitamin B12 Deficiency Test.
Activity Spectrum of the Bioactive Forms
The following table shows the activity spectrum on both of the bioactive forms of B12: methylcobalabim and adenosylcobalamin.
| Form | Site of Action | Activity Spectrum | Symptoms of Deficiency |
| Methylcobalamin | Cell plasma, nerves, brain | Neurotransmitters, gene regulation , regeneration and protection of the nerves and brain, blood formation, vision | Depression, psychological problems, nerve damage, dementia, anaemia, vision disorders, chronic fatigue, exhaustion |
| Adenosylcobalamin | Mitochondria, nerves | Cell energy, brain development, hydration, growth, muscle development | Chronic fatigue, lethargy, weight loss, muscle weakness, developmental disabilities, digestive disorders |
Cyanocobalamin – Synthetic Vitamin B12
As we have discussed, for many years B12 supplements mainly contained cyanocobalamin, a synthetic form of B12 that is not directly bioactive and is only found naturally in the body or diet in minor traces. Cyanocobalamin is however very straightforward and cheap to manufacture – and is particularly stable.
Cyanocobalamin has been well researched and has proven to be very effective and well utilised in the body, in practice. It has been used very successfully for many years in B12 therapy to treat various pathologies. Despite this, cyanocobalamin has been increasingly criticised in recent years, due to the following reasons:
- Toxicity: cyanocobalamin is often claimed to be toxic, as the ‘cyano’ group form the poison cyanide. However, the levels of cyanide resulting from cyanocobalamin are so minimal that the term toxic is hardly applicable here
- Build up in the cells: studies have shown that around 1000 μg of cyanocobalamin accumulates in the cell fluid during therapy with high dosages (2). Yet the consequences are unknown
- Bioavailability: there are four specific metabolic steps necessary to convert cyanocobalamin into one of the coenzyme forms, which is a clear metabolic disadvantage (3)
- Utilisation problems: certain hereditary diseases, as well as metabolic disorders, prevent the conversion of cyanocoalamin into the active B12 forms (4)
- Steals methyl groups: cyanocobalamin requires a methyl group to convert into methylcobalamin, which it takes from the important amino acid S-adenosylmethionine (SAM). Cyanocobalamin thus reduces the SAM level, which is however urgently needed in the body
- Poor storability: finally, cyanocobalamin is inferior to the other B12 forms in terms of absorption. Although cyanocobalamin is more readily absorbed, much of it is excreted through the urine before it can reach the cells
Cyanocobalamin vs Hydroxocobalamin
In comparison to hydroxocobalamin, cyanocobalamin has a significantly worse absorption rate and storability, which is why today mostly hydroxocobalamin is used in B12 injections. Also, one less metabolic step is needed for the conversion of hydroxocobalamin than cyanocobalamin.
Moreover, concerns about exposure to cyanide poisoning are eliminated when hydroxocobalamin is used. Interestingly, hydroxocobalamin is even used to detoxify cyanide. Cyanocobalamin, which is detectable with a normal diet in the body, is usually the result of smoke poisoning or heavy smoking. Especially smokers should thus avoid cyanocobalamin and use other forms of B12 instead – this will keep their exposure to cyanide down, and will even help with detoxification.
Hydroxocobalamin is also an effective scavenger of nitrogen oxides (nitrogen radicals) responsible for the so-called nitrosative stress involved in the development of many diseases.
Cyanocobalamin vs Methylcobalamin
Today there are evermore supplements available that contain methylcobalamin B12. This form can be used directly in the body, without need for conversion, and is better utilised than cyanocobalamin (5).
At comparable oral doses almost identical B12 concentrations were initially detected in the blood serum. However, whereas in the case of cyanocobalamin, large quantities of unused B12 were excreted; methylcobalamin increased cellular levels of B12 and filled the body’s store of the vitamin.
In addition, with methylcobalamin some positive health effects can be achieved that are not possible with cyanocobalamin. For example, in animal studies, methylcobalamin significantly prolonged the survival of mice with cancer, while cyanocobalamin was completely ineffective (6).
This is probably explained by the fact that S-adenosylmethionine (SAM), which is important for many epigenetic processes, is regenerated by methylcobalamin, whereas cyanocobalamin reduces it (as we explored above). Methylcobalamin B12 has also been shown to be superior in tackling sleep disorders, since it is believed to influence melatonin synthesis; whereas cyanocobalamin does not have this effect (7).
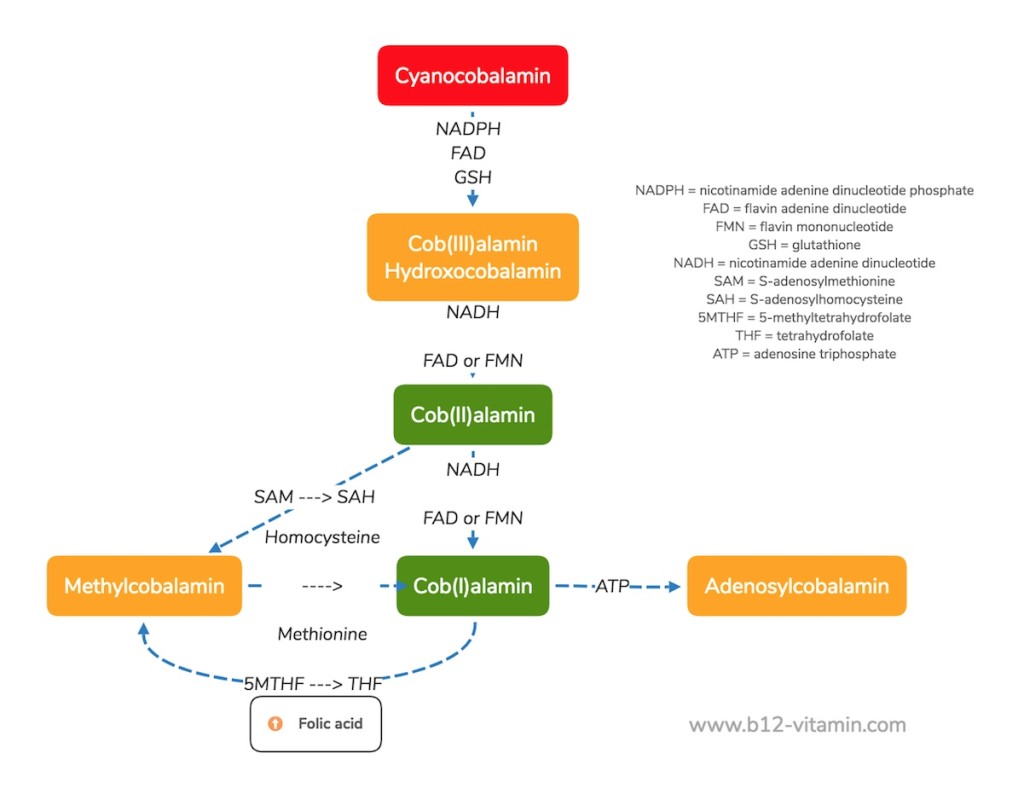
Conversion of the Vitamin B12 Forms
The following graphic shows the conversion steps required for the various vitamin B12 forms:
The Most Well-Known Vitamin B12 Forms
Apart from the ones we have discussed, some other forms of vitamin B12 are known. The following table gives an overview of all of those currently under investigation:
| Form | Other Names/Abbreviations | Description |
| Aquocobalamin | Aquacobalamin, vitamin B12a | B12 + water (H2O), occurs in the body as an intermediate product |
| Hydroxocobalamin | Hydroxycobalamin, vitamin B12b, OH-Cbl | B12 + hydroxy group (OH), produced by microorganisms, occurs in the body and foods |
| Cyanocobalamin | CN-Cbl | B12 + cyano group (CN), synthetic cobalamin, occurs naturally in minor traces |
| Nitritocobalamin | Vitamin B12c | B12 and nitrogen dioxide (NO2) |
| Nitrosocobalamin | B12 and nitrogen monoxide (NO) | |
| Sulfitocobalamin | B12 and sulphur trioxide (SO3) | |
| Methylcobalamin | Methyl-B12, Met-Cbl | B12 + methyl group (CH3), biologically active coenzyme, occurs in the body and foods |
| Adenosylcobalamin | Coenzym B12b, Ado-Cbl, dibencozide | B12 + 5′ desoxyadenosyl (C10H13N5O3), biologically active coenzyme, occurs in the body and foods |
| Glutathionylcobalamin | GS-Cbl | B12 + glutathione, transient precursor of coenzyme, probably has a central role in anti-oxidative and anti-inflammatory processes and in the regulation of NO syntheses (8,9) |
Vitamin B12 – One Vitamin, Many Effects
Not all forms of vitamin B12 are the same. The metabolisation of each form is quite different and the effects differ considerably. While cyanocobalamin has proved effective in preventing vitamin B12 deficiency, there is increasing evidence that the coenzyme B12 forms have a distinct advantage and better spectrum of action in many specialised applications. They do not share the drawbacks of cyanocobalamin, but seem to have significant benefits.
Hydroxocobalamin has certain advantages, especially with its detoxification effect and excellent storability, which helps to ensure a long-lasting B12 supply. This form is also easier for the body to utilise than cyanocobalamin.
Intuitively, it makes sense to assume that the B12 forms found naturally in food are exactly those our body needs. When purchasing supplements, the three natural forms and especially the coenzyme forms should be given preference where possible.
What is more, the vitamin B12 forms we have discussed above do not work alone in the body, but are part of a large complex of B vitamins and operate in combination with a number of other vitamins and minerals. To read more on this, see our article: Vitamin B Complex.
Sources:
- A.G. Freeman Cyanocobalamin – a case for withdrawal: discussion paper. J R Soc Med. Nov 1992; 85(11): 686–687.
- Gimsing P, Hippe E, Helleberg-Rasmussen I, et al. Cobalamin forms in plasma and tissue during treatment of vitamin B12 deficiency. Scand J Haematol 1982;29:311-318
- Pezacka E, Green R, Jacobsen DW. Glutathionylcobalamin as an intermediate in the formation of cobalamin coenzymes. Biochem Biophys Res Commun. 1990 Jun 15;169(2):443-50. PubMed PMID: 2357215.
- Hans C. Andersson, Emmanuel Shapira, Biochemical and clinical response to hydroxocobalamin versus cyanocobalamin treatment in patients with methylmalonic acidemia and homocystinuria (cblC), The Journal of Pediatrics, Volume 132, Issue 1, January 1998, Pages 121-124, ISSN 0022-3476, http://dx.doi.org/10.1016/S0022-3476(98)70496-2.
- Okuda K, Yashima K, Kitazaki T, Takara I. Intestinal absorption and concurrent chemical changes of methylcobalamin. J Lab Clin Med. 1973 Apr;81(4):557-67. PubMed PMID: 4696188.
- Tsao C, S, Myashita K, Influence of Cobalamin on the Survival of Mice Bearing Ascites Tumor. Pathobiology 1993; 61:104-108
- Masayuki Ikeda, Makoto Asai, Takahiro Moriya, Masami Sagara, Shojiro Inoué, Shigenobu Shibata, Methylcobalamin amplifies melatonin-induced circadian phase shifts by facilitation of melatonin synthesis in the rat pineal gland, Brain Research, Volume 795, Issues 1–2, 8 June 1998, Pages 98-104, ISSN 0006-8993, http://dx.doi.org/10.1016/S0006-8993(98)00262-5.
- Carmen Wheatley Cobalamin in inflammation III — glutathionylcobalamin and methylcobalamin/adenosylcobalamin coenzymes: the sword in the stone? How cobalamin may directly regulate the nitric oxide synthases. Journal of Nutritional and Environmental Medicine 2007 16:3-4, 212-226 doi=10.1080%2F13590840701791863
- Catherine S. Birch, Nicola E. Brasch, Andrew McCaddon, John H.H. Williams, A novel role for vitamin B12: Cobalamins are intracellular antioxidants in vitro, Free Radical Biology and Medicine, Volume 47, Issue 2, 15 July 2009, Pages 184-188, ISSN 0891-5849, http://dx.doi.org/10.1016/j.freeradbiomed.2009.04.023.
- J. van Kapel, L.J.M. Spijkers, J. Lindemans, J. Abels, Improved distribution analysis of cobalamins and cobalamin analogues in human plasma in which the use of thiol-blocking agents is a prerequisite, Clinica Chimica Acta, Volume 131, Issue 3, 15 July 1983, Pages 211-224, ISSN 0009-8981
- Thakkar, K., & Billa, G. (2015). Treatment of vitamin B12 deficiency–Methylcobalamine? Cyancobalamine? Hydroxocobalamin?—clearing the confusion. European journal of clinical nutrition, 69(1), 1-2.